News for Industry

Stem Cells cure COVID lungs in severely ill ventilated patients (CBS)
(Miami, FL) In a groundbreaking study, Dr. Camillo Ricordi and his team of researchers at the University of Miami utilized stem cells from umbilical cord tissue to cure COVID patients on ventilators.
Industry Updates from the Field of Stem Cell Research and Regenerative Medicine in August 2020 (Future Medicine)
(London, UK) This review article highlights industry updates in the field of stems of research and regenerative medicine through August 2020. Sections include business development, products, clinical trials as well as regulations, approvals and acquisitions.
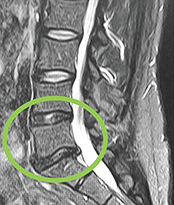
VAST Clinical Trial: Safely Supplementing Tissue Lost to Degenerative Disc Disease (International Journal of Spine Surgery)
(Edmond, OK) Vivex Biologics just published a first ever study showing the results when treating patients with VIA Disc, which is a non-surgical, injectable treatment option for patients suffering from chronic lower back pain related to degenerative disc disease.
The World’s Largest Stem Cell Biobank Launched (Medical Xpress)
(Lund, SE) The world’s largest biobank was launched at Lund University in Sweden. Banking will include stem cells from both healthy and effective individuals for further research, treatment and eventual prevention of diseases.
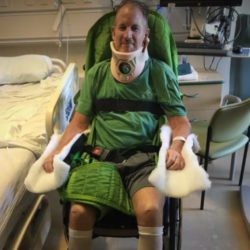
New Medical Innovation Helps Man to Walk Again (Good Morning America)
(Rochester, MN) Scientists and clinicians from the Mayo Clinic used stem cells to help a paralyzed man walk again, as explained by a man with a message and deep personal interest in curing paralysis.

Warning Over Controversial Stem Cell Clinics And Unapproved Treatments (NBC News)
(New York, NY) Many unqualified stem cell clinics and physicians are making false claims of cure for a number of conditions without scientific study or supporting data. This news segment helps patients identify qualified physicians and stem cell centers.

Fujifilm Cellular Dynamics Prepares to Open ‘Cleanroom’ Facility (Kenosha News)
(Madison, WI) Fujifilm Cellular Dynamics announced their opening of a $21 million “cleanroom” to manufacture stem cells for human clinical trials.
GenCure Announces Breakthrough First Run of 80-Liter Bioreactor (GlobeNews Wire)
(San Antonio, TX) GenCure’s bio manufacturing team announced a successful production run where human mesenchymal stem cells were expanded in an 80 Liter bioreactor. This type of scalability will help many patients going forward.

California’s Stem Cell Program Faces an Existential Moment — And a Chance for Reform (Los Angeles Times)
(San Francisco, CA) The California Institute of Regenerative Medicine “CIRM” is gearing up for another multibillion-dollar bond raise. President and CEO Maria Millan, MD states that CIRM continues “… to play an extremely important role of boldly investigating early and de-risking these…” stem cell therapy protocols.
